Simultaneous, multiplexed detection of diverse analytes is critical to many research disciplines and applications. Current multiplex detection tools—especially high throughput ones—can typically only target a single analyte or class of analytes at a time [1]. As a result, detecting diverse analytes presents a high-cost barrier that slows medical diagnoses, scientific research, and disease treatment, particularly in low-resource environments [2, 3].
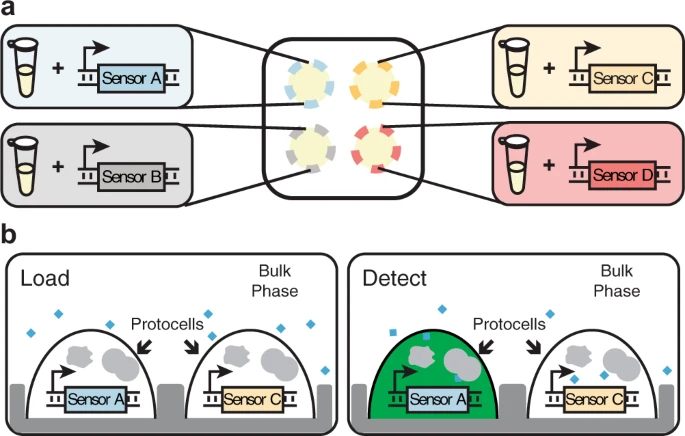
Recently, Zhang et al. developed an innovative solution to this problem: a membraneless protocell system capable of simultaneously detecting analytes such as clinically-relevant ions, small molecules, and nucleic acids [4]. This cell-free expression (CFE) system uses differences in the hydrophilicity of aqueous polymers to create localized regions—droplets—of cell lysate mixtures. This process is referred to as aqueous two phase separation (ATPS). The cell lysate droplets are thus a form of protocells, containing molecular transcription and translation machinery at the necessary concentrations to express sensors that can detect the aforementioned analytes [4].
However, this approach necessitates costly preparation of cell-free lysates. Thus, we developed E.CoDROP, a novel method that eliminates the need for prior preparation of lysates and instead allows rapid production of cell extracts within the ATPS droplets. E.CoDROP promises to build on Zhang et al.'s innovative platform by lowering the cost and resource barrier needed to create these membraneless detection systems, thus moving us closer to highly multiplexed, rapid, low-cost detection tools for both research and diagnostic applications.
CFE systems are becoming increasingly popular and used across synthetic biology for applications such as bioproduction and manipulating gene circuits [5]. Compared to cell-based systems, CFE systems are more streamlined and amenable to analyte detection because there are no cell membranes restricting analyte diffusion. Unlike cells, there is also a reduced risk for engineered organisms to escape into the environment [5].
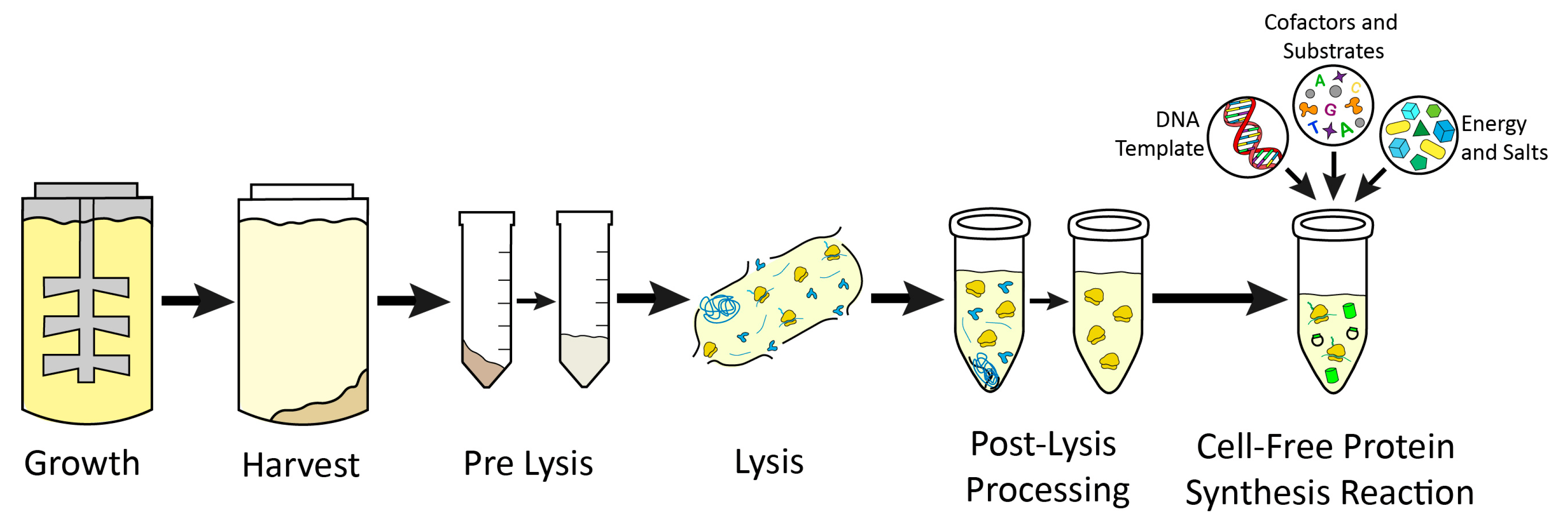
However, these CFE systems are both expensive and technically difficult to produce because they require cell-free lysate, which must be separately produced and purified, while containing the necessary transcription and translation material. Current advancements to simplify cell-free lysate creation include rapid preparation of bacterial lysates using a freeze-thaw cycle [6], centrifugation and sonication [7], and chemical supplements to CFE systems (including amino acids, nucleotides, and energy sources) [6]. This process, however, continues to be resource-intensive, requiring extensive use of laboratory resources that cannot be easily replicated in the low-resource settings where low-cost CFE biosensors could be critical. Thus, there is a clear need for optimizing cell-free lysate production.
We developed a new foundational advance to streamline the production of CFE systems for both in-lab use and versatile diagnostics. Our strategy consists of suspending E. coli cells in the polymer protocells described in Zhang et al. and lysing the cells within these membraneless protocells [4]. This process enables us to functionalize protocells with the released cellular components without first completing high-cost lysate extraction. We first grow Escherichia coli cells to their exponential growth phase, before pellet the cells and resuspend them in an optimized volume of Ficoll. This new Ficoll-cell suspension is vortexed with 35k PEG, reaching final concentrations of 10 w/v% Ficoll and 5 w/v% 35k PEG. These concentrations are based on previous optimizations for stable ATPS droplet formation [4].
Now suspended in the droplets, we induce cell lysis using a lysis gene cassette under an arabinose-inducible pBAD system. The arabinose-inducible system ensures that lysis does not occur before ATPS droplet suspension. When we suspend the cells within droplets, the hydrophilic ficol phase contains growth media with arabinose, which allows the cells within the droplets to grow and then lyse.
This lysis releases all cell contents into the protocell droplets. These contents include the transcription-translation (TX-TL) machinery needed to have functional protocells. Furthermore, because E. coli are genetically tractable and easy to manipulate, we can encode virtually any molecular detection system into the precursor bacterial strain and then release this detection machinery alongside the cell lysate. Going forward, one aim of this project is to verify that our droplets can act as a functional CFE system with both TX-TL machinery and detection components.
Additionally, in order to test a potential avenue for further exploration, we computationally modeled a system where lysis is controlled by bacterial quorum sensing as an alternate to arabinose induction. Our model suggests that this theoretical control system would cause bacteria to automatically lyse once they reach sufficiently high concentrations within the protocells, leading to autonomous formation of cell-free lysate inside the droplets.
Our novel method for cell lysate production promises to enable production of rapid and low-cost tools for lab analysis and point-of-care diagnostics, replacing the expensive cell-free lysate and supplements in existing detection systems. This foundational advance will facilitate the broader adoption of multiple modality detection in CFE systems by enhancing accessibility and enabling its integration as a transformative technology in a wide range of contexts.
References:
[1] Dincer, C., Bruch, R., Kling, A., Dittrich, P. S., & Urban, G. A. (2017). Multiplexed Point-of-Care Testing - xPOCT. Trends in biotechnology, 35(8), 728-742. https://doi.org/10.1016/j.tibtech.2017.03.013
[2] Mani, V., Durmus, C., Khushaim, W., Ferreira, D. C., Timur, S., Arduini, F., & Salama, K. N. (2022). Multiplexed sensing techniques for cardiovascular disease biomarkers - A Review. Biosensors and Bioelectronics, 216, 114680. https://doi.org/10.1016/j.bios.2022.114680
[3] Piorino, F., Patterson, A. T., & Styczynski, M. P. (2022). Low-cost, point-of-care biomarker quantification. Current Opinion in Biotechnology, 76, 102738. https://doi.org/10.1016/j.copbio.2022.102738
[4] Zhang, Y., Kojima, T., Kim, G.-A., McNerney, M. P., Takayama, S., & Styczynski, M. P. (2021, September 29). Protocell arrays for simultaneous detection of diverse analytes. Nature News. https://www.nature.com/articles/s41467-021-25989-3
[5] Tinafar, A., Jaenes, K., & Pardee, K. (2019, August 8). Synthetic biology goes cell-free - BMC biology. BioMed Central. https://bmcbiol.biomedcentral.com/articles/10.1186/s12915-019-0685-x
[6] Didovyk, A., Tonooka, T., Tsimring, L., & Hasty, J. (2017). Rapid and Scalable Preparation of Bacterial Lysates for Cell-Free Gene Expression. ACS synthetic biology, 6(12), 2198-2208. https://doi.org/10.1021/acssynbio.7b00253
[7] Guzman-Chavez, F., Arce, A., Adhikari, A., Vadhin, S., Pedroza-Garcia, J. A., Gandini, C., Ajioka, J. W., Molloy, J., Sanchez-Nieto, S., Varner, J. D., Federici, F., & Haseloff, J. (2022). Constructing cell-free expression systems for low-cost access. ACS Synthetic Biology, 11(3), 1114-1128. https://doi.org/10.1021/acssynbio.1c00342
[8] Gregorio, N. E., Levine, M. Z., & Oza, J. P. (2019). A User's Guide to Cell-Free Protein Synthesis. Methods and Protocols, 2(1), 24. MDPI AG. Retrieved from http://dx.doi.org/10.3390/mps2010024