There are many nations today facing an aging population. Old age brings about numerous
ailments, leading to a decline in the quality of life for those affected. Our project aims
to prevent the occurrence of a specific illness, gout, by utilizing a biologically
engineered medicine in the form of an easy-to-use bottle of liquid medication that is
suitable for all age groups. Gout is a metabolic disease characterized by acute arthritis
attacks and chronic arthritis symptoms. In addition to arthritis attacks, gout may be
accompanied by other symptoms such as fatigue, skin redness, and the formation of nodules[
T. Neogi, Clinical practice. Gout. N Engl J Med 364, 443-452 (2011).]. Gout symptoms usually
appear at night or early in the morning. Patients may experience severe joint pain and
swelling. These symptoms usually affect the big toe, but can also affect other joints, such
as knees, wrists, fingers and elbows. Severe gout can cause patients to lose mobility, while
complications can lead to permanent impairment and disability.
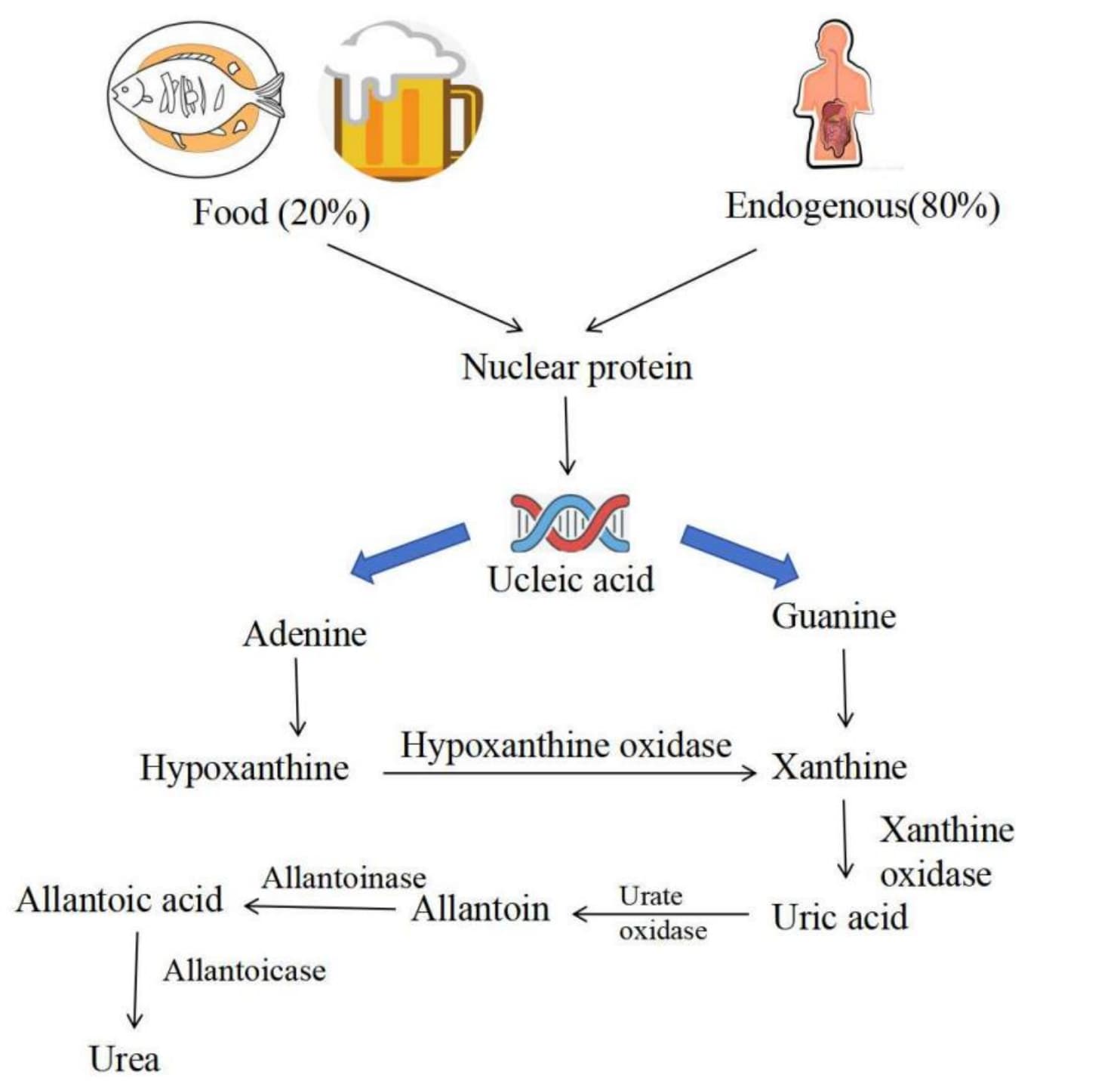
Gout is primarily caused by high levels of uric acid in the body, namely by the condition
known as hyperuricemia[ Y. Zhu, B. J. Pandya, H. K. Choi, Prevalence of gout and
hyperuricemia in the US general population: the National Health and Nutrition Examination
Survey 2007-2008. Arthritis Rheum 63, 3136-3141 (2011).]. Uric acid is a waste product
produced by the metabolism of purines in food. Most of the uric acid is excreted through the
kidneys. However, if the body cannot effectively dispose of uric acid, uric acid levels will
rise, forming urate crystals which are deposited in the joints and the surrounding soft
tissues, causing pain and an inflammatory response, which then leads to joint pain and
swelling. According to research reports, in areas with higher living standards, about 15% of
the population have hyperuricemia, and they are all at risk of gout[ G. Singh, B. Lingala,
A. Mithal, Gout and hyperuricaemia in the USA: prevalence and trends. Rheumatology (Oxford)
58, 2177-2180 (2019).]. There are two main reasons for increased uric acid levels: First,
dietary factors. Intake of purine-rich foods (such as offal, seafood, red meat, etc.) or
high-sugar diets will increase the production of purines in the body, which in turn
increases the production of uric acid. ; The second is genetic factors. Gout is often
related to family inheritance. Some gene mutations can affect the metabolism and excretion
of uric acid, causing uric acid to accumulate in the body[ T. J. Major, N. Dalbeth, E. A.
Stahl, T. R. Merriman, An update on the genetics of hyperuricaemia and gout. Nat Rev
Rheumatol 14, 341-353 (2018).],[ Z. W. Zhou et al., Polymorphisms in GCKR, SLC17A1 and
SLC22A12 were associated with phenotype gout in Han Chinese males: a case-control study. BMC
Med Genet 16, 66 (2015).]. In addition, some diseases and drugs may also cause gout, such as
kidney disease, metabolic disease, hypertension, diabetes, cardiovascular disease, etc. The
use of certain medications, such as diuretics and aspirin, can also increase the risk of
gout.
The goals of gout treatment are to relieve pain, prevent joint damage, control uric acid
levels, and prevent recurrence. At present, there are mainly four types of methods used for
the treatment of gout: First, drug treatment, including non-steroidal anti-inflammatory
drugs, steroids, indomethacin and other analgesics, as well as diuretics, uric acid
synthesis inhibitors and uric acid excretion promoters and other drugs; the second is
lifestyle intervention, including weight control, diet adjustment, and increasing exercise.
In particular, gout patients should control their intake of meat, alcohol, and high-purine
foods, and increase their intake of low-fat dairy products, fruits, and vegetables. The
third option is non-drug treatment, including applying ice to affected areas, elevating the
affected limb, resting and protecting joints. The fourth choice is surgical treatment, such
as joint puncture , fluid extraction and surgical removal of urate deposits in the joints.
Although there are many options for treating gout, there are still some noticeable
drawbacks. For example, many medications have side effects. NSAIDs can cause
gastrointestinal bleeding and kidney problems, while diuretics can cause electrolyte
imbalances and dehydration. Long-term use of certain drugs may lead to drug tolerance and
increase the risk of adverse reactions. For some people, changing eating habits can be
difficult and difficult for patients to adhere to. In addition, surgery itself has risks,
such as infection and post-operative pain.
Currently, research on gout is very active worldwide, involving etiology, prevention,
treatment, and complications. Etiological research mainly focuses on uric acid metabolism
and inflammation. Studies have found that the onset of gout is related to many factors such
as heredity, environment, and lifestyle. Research on prevention shows that controlling diet
and lifestyle habits, reducing body weight, and limiting alcohol intake can reduce the risk
of disease. In addition, preventive drug research for high-risk groups is also underway.
Studies have shown that hyperuricemia is closely related to the incidence of gout, and
reducing uric acid levels can prevent and treat gout[ T. Bardin, P. Richette, Impact of
comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC
Med 7 T. J. Major, N. Dalbeth, E. A. Stahl, T. R. Merriman, An update on the genetics of
hyperuricaemia and gout. Nat Rev Rheumatol 14, 341-353 (2018).
8Rebai Y,Wagner L,Gnaien M, et al. Escherichia coli Nissle 1917 Antagonizes Candida albicans
Growth and Protects Intestinal Cells from C. albicans -Mediated Damage[J].
Microorganisms,2023,11(8).
9Kleta, S., et al. (2016). “Clinical practice: the probiotic Escherichia coli strain Nissle
1917 (EcN)-from bench to bedside.” European journal of pediatrics, 175(2), 151-162.
10Henker, J., et al. (2007). “Multicenter, double-blind, placebo-controlled trial comparing
2 different formulations of heat-killed Escherichia coli in patients with ulcerative
colitis.” American Journal of Gastroenterology, 102(10), 2236-2249.
11Sonnenborn, U. (2016). “Escherichia coli strain Nissle 1917–a valuable resource in basic
and applied science and therapeutics.” Microbial ecology in health and disease, 27(sup1),
29758.
12Tursi, A., et al. (2007). “Effects of mesalazine, antibiotics and probiotics on
pro-inflammatory cytokines, nitrites and myeloperoxidase in ulcerative colitis.” World
Journal of Gastroenterology, 13(23), 3168-3172.
]. Research on treatment mainly focuses on drug treatment and non-drug treatment. Medication
treatments include uric acid-lowering drugs, nonsteroidal anti-inflammatory drugs, and
steroids. Non-drug treatments include changes in diet and lifestyle. Research on
complications focuses more on the relationship between gout and its complications, and how
to reduce the risk of complications. Overall, current research on gout is very extensive and
active worldwide.
Nissle 1917 is a specific strain of Escherichia coli (E. coli) bacteria that has been
extensively researched for its beneficial effects on the gastrointestinal system9. It was
first isolated in 1917 by Dr. Alfred Nissle and has since been used as a probiotic to
promote gut health10. Nissle 1917 has been shown to have antimicrobial properties, help
restore the balance of gut flora, and support the immune system11. It is considered safe for
human consumption and has been used in various medical and research applications12.
Though developments for better treatment are already underway, people still suffer from
gout. When we learned about the suffering that is inflicted upon individuals by this disease
on such a large scale, we made it our goal to develop treatments for it.