Engineering Design-Build-Test-Learn
Our ultimate goal is to produce the bioplastic PHB…
…but how? Where do we start?
Like all engineering, Bio-Barbie uses scientific principles to engineer biological tools to synthesize bioplastic.
Overview
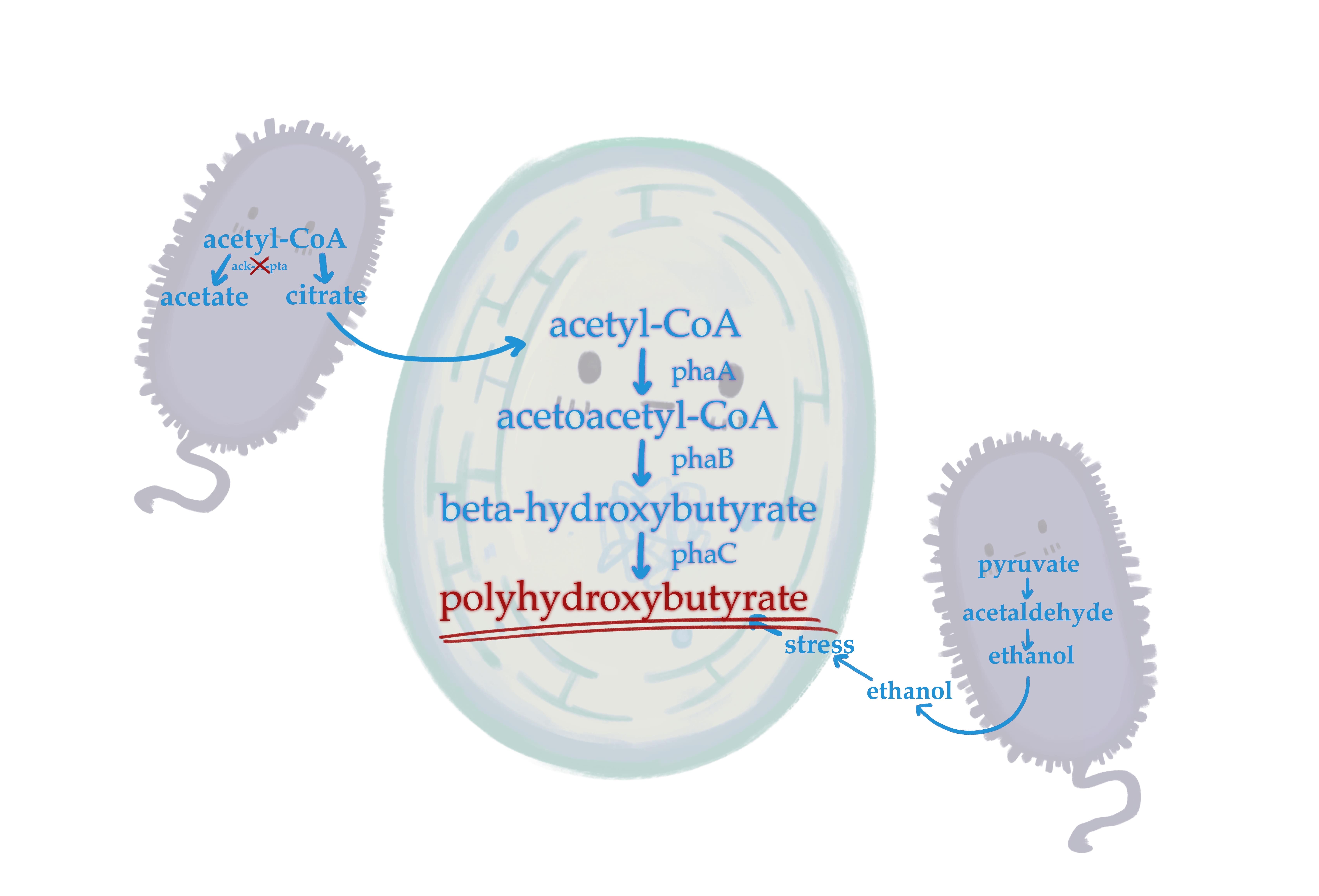
Polyhydroxybutyrate (PHB) is one of the most well-studied bioplastics. PHB is a common type of polyhydroxyalkanoate (PHA), a class of molecules produced for energy storage when the microorganism is under stress. Our chassis for PHB production, C. reinhardtii, naturally makes a low amount of PHB under such stressful conditions, such as nutrient starvation (Cassuriaga et al. 2020). In co-culture with E. coli, we look to push PHB production even further (Yamada et al. 2023).
Our project increases PHB production at the beginning, middle, and end of PHB synthesis. We accomplish this by:
- Genetically modifying C. reinhardtii to express phaA, phaB, and phaC to increase PHB production
- Inducing stress in C. reinhardtii with an E. coli strain genetically modified to produce ethanol, a stress factor
- Inactivating ackA-pta to increase acetyl-CoA available for PHB synthesis
PHB Synthesis in C. reinhardtii
To increase the amount of PHB produced by C. reinhardtii, we designed three genes: phaA (BBa_K4736005), phaB (BBa_K4736006), and phaC (BBa_K4736007). These genes come from Cupriavidus necator, a soil bacterium previously known as Ralstonia eutropha. We selected these genes because C. necator is able to accumulate PHB up to 90% of its cell dry weight (Obruca et al., 2014). After modifying these genes to be compatible with the RFC 10 standard, they were synthesized by Integrated DNA Technologies (IDT).
To build our construct for expression, we selected the promoter CaMV35S (BBa_J428074) and eukaryotic terminator (BBa_J428081) from the Plants Collection in the 2023 iGEM Kit Plate.
Since our lab has never successfully transformed a microalgae, we also designed an expression construct for mCherry (BBa_K4736008), a reporter gene that is compatible with C. reinhardtii, using the same promoter and terminator. This allows us to easily and quickly screen our algae culture for successful transformants.
We have successfully built these constructs through a series of restriction digests, gel extractions, ligations, transformations, and mini-preps, and we have transformed them into E. coli to test if our PHB genes are functioning as expected. We expect to have completed at least one round of testing by the 2023 iGEM Jamboree. Once we have confirmed that our genes are working as expected, we will transform them into C. reinhardtii and test for increased PHB production. Based on these results, we may modify our design or experiment with different promoters and culture conditions to maximize PHB production.
Biosafety System Design
Introduction:
The increasing applications of synthetic biology emphasize the requirement for robust biosafety measures. Our project exploits the symbiotic co-culturing of E. coli and C. reinhardtii. To ensure that these genetically modified organisms don't pose environmental threats upon potential release, we have implemented a two-fold biosafety strategy involving a toxin-antitoxin system in E. coli and an auxotrophy mechanism in C. reinhardtii.
E. coli Kill Switch Design using the Toxin-Antitoxin System:
1. Gene Selection:
Toxin: RelE, a ribonuclease, that upon expression, inhibits translation by cleaving mRNAs in the ribosomal A-site.
Antitoxin: RelB, which binds and neutralizes the toxic effect of RelE, produced at a high level when induced.
2. Promoter, Regulator & Inducer Selection for Antitoxin Production:
Promoter for Antitoxin: Ptrc promoter – A hybrid of trc and lac promoters. This promoter is renowned for its tight regulatory control and is often used in IGEM, offering low basal expression levels in the absence of an inducer, ensuring that the antitoxin doesn't unnecessarily burden the bacterial metabolism. However, upon the introduction of the inducer IPTG, the Ptrc promoter provides strong and robust expression, ensuring a sufficient amount of RelB antitoxin is produced to counteract the toxin.
Regulator: The LacI protein plays a pivotal role in the functionality of the Ptrc promoter. Without the inducer, LacI binds to Ptrc, preventing transcription. This regulation ensures that the antitoxin isn't produced in environments where it's not needed.
Inducer: IPTG is used as the synthetic inducer. When introduced into the environment, IPTG binds to LacI, causing a conformational change that prevents it from binding to the Ptrc promoter. This, in turn, induces the transcription of the downstream genes, in this case, the RelB antitoxin.
3. Promoter for Toxin Production:
Promoter for Toxin: PJ23101 promoter - This is a well-established constitutive promoter widely used in synthetic biology for E. coli applications. Being constitutive, it ensures a steady and consistent level of gene expression without the requirement of any inducers. By employing the PJ23101 promoter to control the expression of the toxin gene, we're ensuring a continual, moderate level production of the toxin. This level is counteracted by the high expression of antitoxin when in the laboratory condition with the inducer present. However, in the event of an escape, the reduced expression of antitoxin combined with the consistent production of toxin ensures the kill switch's activation.
4. Potential Impact of IPTG on C. reinhardtii:
Concern: Though IPTG is inert in many cellular contexts, we need to consider the potential effects it may have on C. reinhardtii growth or physiology.
Experimental Validation: To address this, an experiment can be designed where C. reinhardtii cultures are exposed to varying concentrations of IPTG, encompassing our working concentration. By monitoring growth rates, chlorophyll content, and other physiological markers, we can assess any potential negative impacts of IPTG on the health of algae.
5. Operational Mechanism:
In Laboratory Conditions:
Activation of Antitoxin Production:
In the presence of IPTG in the culture medium, the Ptrc promoter, controlling the antitoxin gene RelB, becomes highly activated. This leads to a robust expression of the antitoxin, RelB. Due to the strong induction provided by IPTG, the amount of RelB expressed is sufficient to neutralize the effects of the concurrently produced toxin, RelE, ensuring the E. coli cells remain healthy and viable.
Consistent Toxin Production:
The RelE toxin is expressed under the control of the PJ23101 promoter, a moderate-strength constitutive promoter. This guarantees a steady, consistent production of the toxin irrespective of the presence or absence of IPTG. However, in the lab conditions with IPTG present, the high level of RelB antitoxin counteracts this toxin, allowing the bacterial cells to grow normally.
Post-escape Scenario:
Antitoxin Production Ceases:
In the event the E. coli cells escape into an environment devoid of IPTG, the Ptrc promoter driving RelB expression is not activated. This means the high-level production of RelB antitoxin ceases, dramatically reducing its concentration in the bacterial cell over time.
Continual Toxin Challenge:
The consistent expression of the toxin, RelE, due to the PJ23101 promoter continues irrespective of the external environment. Without sufficient levels of its neutralizing antitoxin, RelB, the harmful effects of RelE start manifesting, leading to bacterial cell death. This serves as an effective safety mechanism, ensuring the genetically modified bacteria cannot survive outside the controlled lab conditions.
Auxotrophy Mechanism in C. reinhardtii Using SDH Inhibition:
1. Background:
The tricarboxylic acid cycle (TCA cycle) is central to cellular metabolism in both prokaryotic and eukaryotic organisms. Disrupting a critical step within this cycle can have profound consequences on an organism's viability. For our kill switch strategy in the eukaryotic algae, we're targeting Succinate Dehydrogenase (SDH) in the TCA cycle, aiming to inhibit its function. This unique approach harnesses the inherent metabolic processes of the cell, making it a feasible and innovative strategy.
Our attention is on the inhibition of Succinate Dehydrogenase (SDH), a crucial enzyme in the TCA cycle that oversees the conversion of succinate to fumarate. Oxaloacetate (OAA) has been identified as a competitive inhibitor of SDH. Specifically, OAA binds with a sulfhydryl group of SDH, effectively abolishing enzymatic activity. This bond subsequently evolves into a thiohemiacetal, making the inhibition almost irreversible. With the elevated levels of OAA, the activity of SDH gets inhibited, obstructing the conversion of succinate to fumarate.
To naturally increase OAA levels within the cell, we turn our focus to Phosphoenolpyruvate Carboxylase (PEPC). PEPC facilitates the conversion of phosphoenolpyruvate (PEP) to OAA. By amplifying the expression of PEPC, we anticipate a surge in OAA concentrations, culminating in the effective inhibition of SDH.
2. Gene Selection:
Phosphoenolpyruvate Carboxylase (PEPC): We've chosen the PEPC gene for our approach. PEPC is an enzyme responsible for converting phosphoenolpyruvate (PEP) to oxaloacetate (OAA). By overexpressing this gene, we aim to increase the intracellular concentrations of OAA. Elevated OAA levels will act as a competitive inhibitor for SDH, thereby affecting the TCA cycle and disrupting cellular metabolism.
3. C4-like Pathway in C. reinhardtii:
C. reinhardtii, although primarily operating through the C3 photosynthetic pathway, has been found to have the components for a C4-like pathway. PEPC plays a role in this pathway, converting PEP to OAA. By manipulating this pathway and enhancing the activity of PEPC, we can strategically increase OAA levels within the cell. This provides a foundation for our kill switch strategy, allowing us to leverage the native metabolic pathways of the algae for our biosafety objective.
4. Promoter Selection for PEPC Overexpression:
PsaD promoter: The PsaD promoter from C. reinhardtii is one of the most commonly used promoters for strong, constitutive expression in this alga. It drives the expression of the PsaD gene, which is a part of the photosystem I complex. Given its robust and consistent expression patterns, it seems suitable for our strategy.
5. Determining Optimal Fumarate Concentration:
Once the transformation has been validated, the next crucial step would be to identify the optimal fumarate concentration in the growth medium. This ensures the algae’s survival in laboratory conditions while guaranteeing effective containment outside. To determine this, we would culture the transformed algae under varying fumarate concentrations, monitoring growth parameters, especially cell density. The concentration that yields the highest growth rate without any observable stress would be considered optimal.
6. Validation After Successful Transformation:
- Gene Expression: Quantitative RT-PCR to measure the mRNA levels of the introduced PEPC gene.
- Protein Expression: Western Blot analysis to confirm the expression of the PEPC protein.
- Growth Curves: Compare the growth of transformed and non-transformed C. reinhardtii in the presence and absence of external fumarate.
7. Operational Mechanism:
In Laboratory Conditions:
- The transformed algae will be cultured in media supplemented with optimal concentrations of fumarate.
- The introduced PEPC gene, driven by the PsaD promoter, will result in elevated OAA levels, inhibiting SDH and causing a disruption in the TCA cycle.
- The external fumarate will bypass the blocked step, allowing the algae to grow.
Post-escape Scenario:
- In an environment without supplemented fumarate, the transformed algae will fail to thrive due to the inhibition of SDH by OAA.
- This will act as a biological containment mechanism, preventing the algae from growing outside controlled conditions.
Parts
Basic Parts:
| Repository Accession Number | Short Name | Description |
|---|---|---|
| BBa_K4736000 | pdc (Pyruvate Decarboxylase) | This is the coding sequence for the enzyme pyruvate decarboxylase, which catalyses the conversion of pyruvic acid to acetaldehyde. When used in conjunction with adhB (BBa_K4736001), it can be used to create ethanol. |
| BBa_K4736001 | AdhB (alcohol dehydrogenase II) | This is the coding sequence for the enzyme alcohol dehydrogenase II, which catalyzes the conversion of aldehydes to alcohols. In our project, we used this gene in conjunction with pdc (BBa_K4736000) to synthesize ethanol from acetaldehyde. |
| BBa_K4736003 | recET (exonuclease VIII) | This is the coding sequence for the enzyme exonuclease VIII, which facilitates homologous recombination of linear DNA between short regions of homology. RecET is analogous to lambda Red recombinase, which also facilitates recombination of short region homology. When paired with regions homologous to a target gene, recET can be used to facilitate a gene knockout. |
| BBa_K4736004 | ackAF-pta knockout | This part is designed to inactivate E. coli's main acetate production pathway ackA-pta. This part includes the gene for ampicillin resistance, flanked by two short regions homologous to the ackAF and ptaR genes, respectively. When transformed as linear DNA into an E. coli strain expressing recET (BBa_K4736003), a gene that facilitates the recombination of linear DNA into E. coli's genome, this construct will replace the ackA-pta pathway with the gene for ampicillin resistance, allowing for easy selection of successfully modified bacteria. The goal of inactivating the acetate production pathway is to increase the amount of acetyl CoA available for PHB synthesis. |
| BBa_K4736005 | phaA | PhaA is the coding sequence for the enzyme acetyl-CoA acetyltransferase. This enzyme catalyzes the conversion of acetyl-CoA into CoA and acetoacetyl-CoA. It is the first step in converting acetyl-CoA into polyhydroxybutyrate (PHB). |
| BBa_K4736006 | phaB | This gene codes for the enzyme acetoacetyl-CoA reductase. This enzyme catalyzes the reduction of acetoacetyl-CoA to β-hydroxybutyrate, the second of three steps for PHB synthesis from acetyl-CoA. |
| BBa_K4736007 | phaC | This gene codes for the enzyme polyhydroxybutyrate polymerase. This enzyme catalyzes the polymerization of β-hydroxybutyrate into polyhydroxybutyrate, or PHB, the last of three steps in the production of PHB. |